Cuesta College, San Luis Obispo, CA
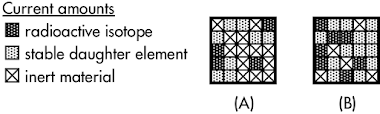 Two samples have certain amounts of a radioactive isotope, an embedded gaseous daughter element, and inert material (which is not involved in the decay process). As determined by radioactive dating, __________ has an older solidification age.
Two samples have certain amounts of a radioactive isotope, an embedded gaseous daughter element, and inert material (which is not involved in the decay process). As determined by radioactive dating, __________ has an older solidification age.(A) sample A.
(B) sample B.
(C) (There is a tie.)
(D) (Cannot be determined.)
Correct answer (highlight to unhide): (A)
The solidification age of a sample (how long ago has it been since it was last molten) is determined by the ratio of decay products to its unstable isotopes. A larger ratio of decay products to unstable isotopes corresponds to a sample with a very old solidification age.
Sample A started with 12 unstable isotope squares when it first solidified (as any gaseous decay products would have been released when it was melted), of which 4 remain today, such that it has 33% of its original radioactivity today. Sample B started with 20 unstable isotope squares when it first solidified, of which 7 remain today, such that it has 35% of its original radioactivity today.
Section 30882
Exam code: quiz07b3Ta
(A) : 4 students
(B) : 1 student
(C) : 3 students
(D) : 0 students
Success level: 60%
Discrimination index (Aubrecht & Aubrecht, 1983): 0.46
No comments:
Post a Comment